I’m pretty sure it seems weird for me to be writing about beauty products but this one in particular is a pretty fascinating product to me. It actually holds a lot of basic chemistry concepts despite being such a simple cosmetic product. Plus I’m also fascinated by it because of its origin. You see, micellar water isn’t a new trend. It’s been around for almost a century already. The trend of micellar water started in early france. I’m not sure when exactly it started but the first company that utilized this concept is bioderma. It came out around 1913 and was followed by an American company in 1993. As to why micellar water became widespread was because France’s was mostly hard water. I don’t have much information on their water sources but seeing as they mostly utilized sea water and deep well water, it could be a factor in their water’s calcium and magnesium content. It could also be attributed to the fact that they have chalky soil in some regions.

This is what happens when soap is used in hard water. It's usually thicker though
Why is oil mixed with water so fascinating?
To understand that, we need to look at its individual key ingredients. The water part, and the oil part.

First is the water which isn’t really just regular water. And if you’ve worked in the lab like me, you’ll know that water isn’t just water because there are so many types of water. There’s deionized water, reverse osmosis water, distilled water, hard water, etc. For this particular product, although most beauty lines use “purified water” what they actually use is soft water (yes, there’s soft water and there’s also hard water). This isn’t harmful, don’t worry 😅😅😅 on the contrary, it’s actually the kind of water that’s good for people because of the lower Calcium and magnesium content in it (It’s opposite with hard water. Do you know that white stuff that builds up in the bathroom if your water is from deep wells? Those are mostly caused by hard water-binding with your soap molecules and it can be pretty irritating). But nowadays, they use other types of water like essence-infused water and whatnot.
For the oil part. I wonder if they taught this in high school but oils and water don’t mix. If you try to mix oil with water, they form these little separations called micelles. This micelle is the very soul of the product since it makes cleansing and moisturizing possible.
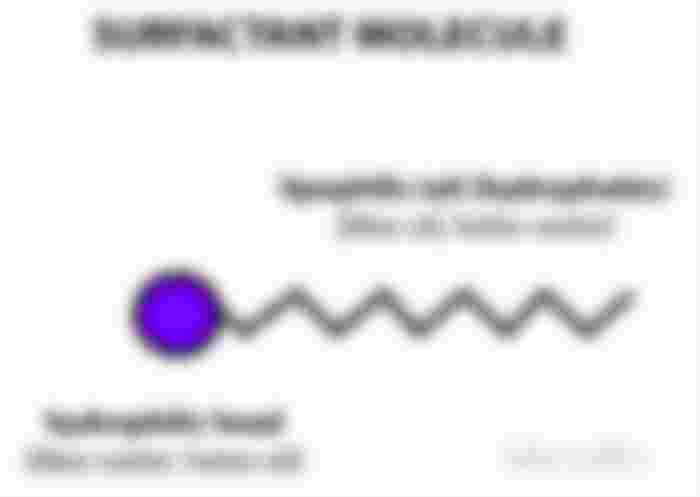
For the basic chemistry of it, we’ll have to look at an oil’s molecular structure. An oil and almost any surfactant molecule (including soaps and detergents) will have similar structures where they have a water-loving (hydrophilic) and water hating (hydrophobic) end.

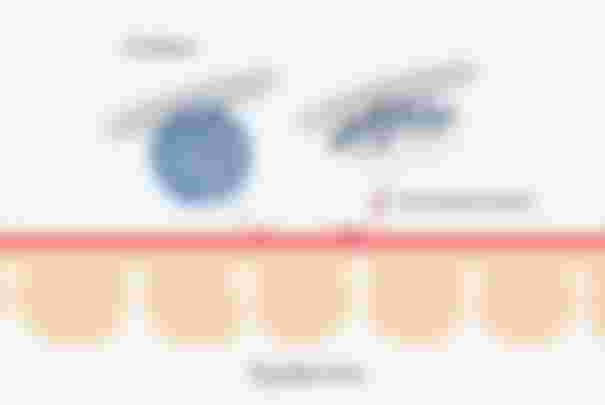
On a mechanistic view, these micelles are ingenious since the hydrophilic ends will face outwards while the hydrophobic ends will stay away from water. Despite the ball figure they make, the hydrophobic end will still be attracted to dirt. And this is where the “like attracts like” concept in chemistry comes in handy. You see, the dirt, make up and oil on your skin are basically hydrophobic. Same constituents so they’ll attract each other.
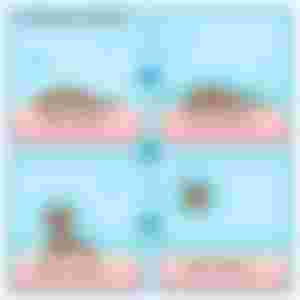
But the difference between soaps and this micellar water is that it doesn’t make your skin dry like what happens when you just rinse your face with just soap. Also, because of this very gentle formulation, it’s good for all skin types, even the highly sensitive ones. And again, that’s because of the soft water they use for it. Of course, additional ingredients also contribute to the moisturizing properties of micellar water like the type of oil they used, the amount of glycerin, triglycerides, lecithin, and a few other ingredients that aren’t irritating to the skin.
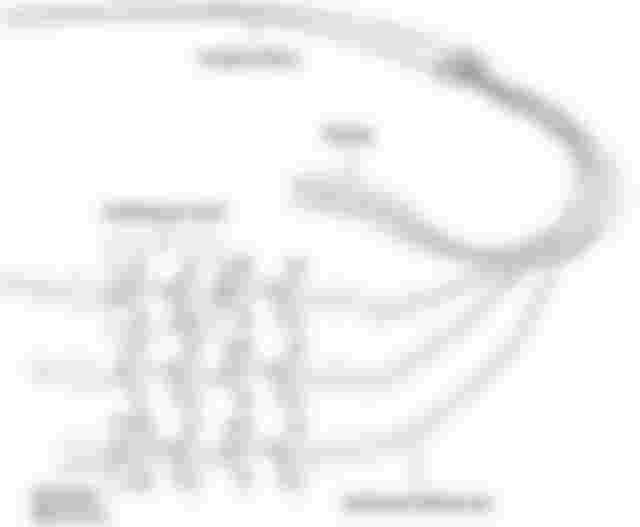
There’s also something funny I found while looking up the ingredients of micellar water. Apparently, it’s not just the product itself that does the cleansing but also in the method of applying 🤣🤣🤣 So cotton pads have a lot of hydrophilic ends in their structures (which is why cotton is so absorbent). That very fact means that the hydrophilic ends of the oil/surfactant molecule have something to bind too. And instead of the oils forming micelles, they leave the hydrophobic ends fully exposed to make dirt/carbon absorption much easier.

All those -OH (hydroxyl) ends are the ones that attract the hydrophilic ends

Sot this is what it essentially looks like on a bigger picture. And this logic kind of makes the surface area in which micellar water reaches larger compared to your soap and water.
It’s funny to me but it does make sense but I’m concerned with the environmental impact of the disposable cotton people use. I hope they do something like inventing reusable cotton pads for makeup.
This is enough of my chemistry rambles for the day XD
Related article:
https://read.cash/@Hanzell/chemistry-for-the-day-kojic-acid-vs-glutathione-vs-collagen-24d03cca



I am amazed on how you explained what this product has. Chemistry at is finest. Ive been using micellar water but no knowledge how it was made or what it has. Well thanks to this article you have. ☺️