In this article I will discuss what titration is all about and the importance of it in the world of science. Before I discuss it I provided glossary of terms because some of the words are technical or will be unfamiliar with the readers.
Terminologies
1. Analyte - an identified solution that has an unknown concentration which is to be determined.
2. Endpoint - a point that signals the complete reaction of acid and base and is also determined through the change in color of the analyte.
3. Titrant - a solution with known concentration and volume that is reacted with the analyte to determine the latter’s concentration.
4. Equivalence point - a point wherein the acid and base are chemically equal in moles.
5. Indicator - a solution that is added to the analyte that signals whether the analyte is acidic or alkaline through the change in color.
6. pH - a measure of the hydrogen ion concentration of a solution and a scale that specifies the acidity and alkalinity of an aqueous solution.
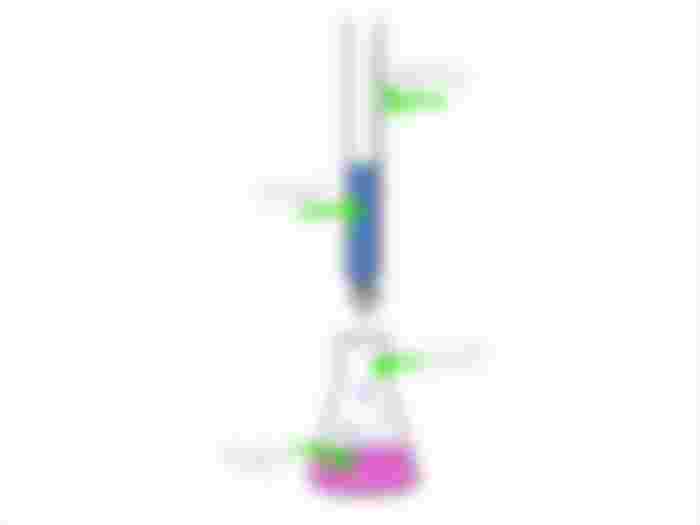
The process of determining the unknown concentration of the identified analyte by slowly adding another solution of known concentration referred to us the titrant is called Titration. In order to know physically, if the analyte reach its endpoint, an indicator such as phenolphthalein (colorless in an acidic solution but is violet in an alkaline) is added to the analyte. For example, if phenolphthalein is used in the titration of base with an acid, the solution would turn into a pale pink color signifying that it has approached the equivalence point.
For instance, if we titrate strong base with strong acid we will be able to determine the concentration and identify how the pH level changes as the addition of acid takes place.


The table above shows the relationship between the added volume of titrant and the pH level of the analyte. In this experiment we used Hydrochloric Acid (HCl) as our titrant while Sodium Hydroxide (NaOH) as the analyte. As you can see as the addition of titrant increases, the pH level decreases, which is rational because the solution become acidic.
You can also use acid as the analyte and base as the titrant because it depends on what solution you want to determine.
Thank you for reading I hope you learned something from this articles.😊
I would like to thank my sponsors @Winona , @Bestie , @Allesh12 for their support, please subscribe them they write good articles.
Please do subscribe @Manasseh and @Lynden25re still new but they will upload their articles soon, I hope you'll also support them. Thank you❤
I'm also glad to join "Get Sponsored !!" community.
The idea of community is to sponsor quality content creators & reward community members for their activities.
Join if you want to "Get Sponsored !!"
Thank you @Ashma for the continuous support! I am motivated to write more under your support💕

Nice job👍👍