The skin is the body's primary barrier against physical insults and microbial pathogens. It represents a unique environment in which immune cells interact with skin cells to maintain tissue homeostasis and induce immune responses.
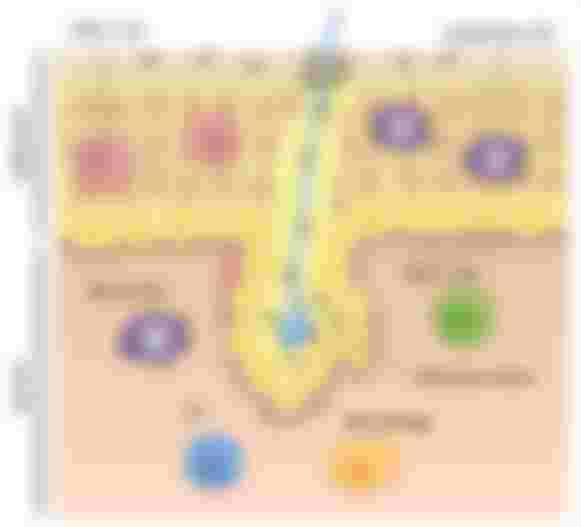
The skin is composed of the epidermis dermis and a subcutaneous fatty region. Commensal bacteria fungi and viruses living on the skin have beneficial effects in the protection against pathogens and in wound healing. The epidermis is composed of highly specialized epithelial cells known as keratinocytes. they are continuously replenished from just one layer of basal keratinocytes, which divide frequently. Dead cells called cornea sites form the outermost layer and are largely responsible for the barrier function of the skin. In the dermis cells known as fibroblasts secrete elastin and collagen fibers that form a dense extracellular matrix. Blood capillaries irrigate the dermis while lymph fluid is drained through lymphatic vessels to lymph nodes specialized immune structures in which immune cells are activated after pathogen encounter. Diverse and functionally specialized immune cells populate the skin.

The epidermis is a specialized subset of dendritic cells called Langerhans cells sample antigen. They project dendrites upward towards the qualified epithelial layer and sample bacterial antigens such as toxins. Langerhans cells appear to be both anti-inflammatory and activator depending on the context. Dendritic cells in the dermis are highly efficient at capturing dead cells and presenting antigens such as viruses other intracellular pathogens or skin-associated self-antigen to t-cells. If dendritic cells are the immune Sentinels T cells are the immune effectors. healthy skin contains more than twice the number of T-cells found in the blood. Most of them are memory t-cells that have previously encountered antigens and can be rapidly reactivated.
T cells in the epidermis are mostly cd8 t-cells. A subset that becomes cytotoxic and kills target cells upon activation. their long-term residence in the epidermis is mostly disconnected from the circulation. T cells in the dermis are mostly helper cd4 T cells, which have a more modulatory role in the immune response. a variety of other immune cells such as natural killer cells eosinophils, and mast cells are present in the dermis and might be involved in allergic reactions in the skin. Dendritic cells and keratinocytes sense tissue damage such as wounds or cold sore lesions that occur when latent herpes virus reactivates, and they do that through evolutionarily conserved receptors that recognize pathogen-derived molecular patterns or host-derived molecules that are exposed by cell death such as DNA. keratinocytes produce antimicrobial peptides that can kill bacteria directly, inflammatory mediators such as interleukin 1 or il-1, which activate dendritic cells, and chemokines that recruit neutrophils, macrophages, and T cells. Activated dendritic cells migrate to the lymph nodes where they present antigen from the site of infection to naive t-cells priming them to activate and differentiate into effector T cells. activated t-cells return to the skin and kill infected keratinocytes to control viral infection or secrete signals that recruit additional immune effector cells. following viral clearance memory, cd8 t-cells persist in the epidermis to provide immunity for future encounters with the same virus. immune responses can become dysregulated and cause skin disorders such as psoriasis or atopic dermatitis.

Psoriasis is a lifelong inflammatory skin disease characterized by scaly reddish plaques. A combination of environmental and genetic factors confers susceptibility to the disease. physical injury or inflammation can trigger the formation of an acute lesion, the antigenic trigger is unknown but current models propose that stressed keratinocytes might release self DNA, which is complex with an antimicrobial peptide activates dermal plasma site or dendritic cells to secrete high amounts of the antiviral mediator interferon.

Together with pro-inflammatory il-1 alpha released by stressed keratinocytes, interferon activates dermal dendritic cells to promote T cell differentiation. The earliest recognizable change in the affected skin is the accumulation of T cells and dendritic cells around blood vessels in the dermis. an overt lesion occurs when cd8 t-cells dendritic cells and neutrophils infiltrate the epidermis. specialized subsets of T cells secrete soluble mediators like interferon-gamma and il-17, which stimulate the proliferation of keratinocytes and this produces a marked thickening of the epidermis. Signals from the proliferating keratinocytes act as a chemoattractant for infiltrating neutrophils. Crosstalk between immune cells keratinocytes and dermal cells thus contribute to tissue remodeling and amplification of this dysregulated immune response. Without treatment acute psoriatic lesions become chronically psoriatic lesions, genetic studies have identified psoriasis associated susceptibility genes, some of them linking th17 cells the subset of T cells that produce il-17 to psoriasis pathogenesis.
Thus cells in the skin exert important roles in maintaining the barrier function against pathogens, but can also become activated by self-antigen or harmless antigen to cause autoimmunity or allergies