What is fertiliser?

A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin (other than liming materials) that is applied to soil or to plant tissues to supply one or more plant nutrients essential to the growth of plants. Many sources of fertilizer exist, both natural and industrially produced.
In the later half of the 20th century, increased use of nitrogen fertilizers (800% increase between 1961 and 2019) have been a crucial component of the increased productivity of conventional food systems (more than 30% per capita). According to the IPCC Special Report on Climate Change and Land, these practices are key drivers of global warming.
History
World population supported with and without synthetic nitrogen fertilizers.

Management of soil fertility has been the preoccupation of farmers for thousands of years. Egyptians, Romans, Babylonians, and early Germans are all recorded as using minerals and or manure to enhance the productivity of their farms.[1] The modern science of plant nutrition started in the 19th century and the work of German chemist Justus von Liebig, among others. John Bennet Lawes, an English entrepreneur, began to experiment on the effects of various manures on plants growing in pots in 1837, and a year or two later the experiments were extended to crops in the field. One immediate consequence was that in 1842 he patented a manure formed by treating phosphates with sulfuric acid, and thus was the first to create the artificial manure industry. In the succeeding year he enlisted the services of Joseph Henry Gilbert, with whom he carried on for more than half a century on experiments in raising crops at the Institute of Arable Crops Research.
The Birkeland–Eyde process was one of the competing industrial processes in the beginning of nitrogen based fertilizer production.This process was used to fix atmospheric nitrogen (N2) into nitric acid (HNO3), one of several chemical processes generally referred to as nitrogen fixation. The resultant nitric acid was then used as a source of nitrate (NO3−). A factory based on the process was built in Rjukan and Notodden in Norway, combined with the building of large hydroelectric power facilities.
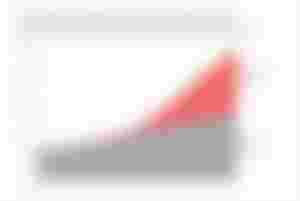
The 1910s and 1920s witnessed the rise of the Haber process and the Ostwald process. The Haber process produces ammonia (NH3) from methane (CH4) gas and molecular nitrogen (N2). The ammonia from the Haber process is then converted into nitric acid (HNO3) in the Ostwald process.The use of synthetic nitrogen fertilizers has increased steadily in the last 50 years, rising almost 20-fold to the current rate of 100 million tonnes of nitrogen per year.The development of synthetic nitrogen fertilizer has significantly supported global population growth — it has been estimated that almost half the people on the Earth are currently fed as a result of synthetic nitrogen fertilizer use.The use of phosphate fertilizers has also increased from 9 million tonnes per year in 1960 to 40 million tonnes per year in 2000. A maize crop yielding 6–9 tonnes of grain per hectare (2.5 acres) requires 31–50 kilograms (68–110 lb) of phosphate fertilizer to be applied; soybean crops require about half, as 20–25 kg per hectare.Yara International is the world's largest producer of nitrogen-based fertilizers.
Controlled-nitrogen-release technologies based on polymers derived from combining urea and formaldehyde were first produced in 1936 and commercialized in 1955.The early product had 60 percent of the total nitrogen cold-water-insoluble, and the unreacted (quick-release) less than 15%. Methylene ureas were commercialized in the 1960s and 1970s, having 25% and 60% of the nitrogen as cold-water-insoluble, and unreacted urea nitrogen in the range of 15% to 30%.
In the 1960s, the Tennessee Valley Authority National Fertilizer Development Center began developing sulfur-coated urea; sulfur was used as the principal coating material because of its low cost and its value as a secondary nutrient. There is usually another wax or polymer that seals the sulfur; the slow-release properties depend on the degradation of the secondary sealant by soil microbes as well as mechanical imperfections (cracks, etc.) in the sulfur. They typically provide 6 to 16 weeks of delayed release in turf applications. When a hard polymer is used as the secondary coating, the properties are a cross between diffusion-controlled particles and traditional sulfur-coated.
ClassificationEdit
Fertilizers are classified in several ways. They are classified according to whether they provide a single nutrient (e.g., K, P, or N), in which case they are classified as "straight fertilizers." "Multinutrient fertilizers" (or "complex fertilizers") provide two or more nutrients, for example N and P. Fertilizers are also sometimes classified as inorganic (the topic of most of this article) versus organic. Inorganic fertilizers exclude carbon-containing materials except ureas. Organic fertilizers are usually (recycled) plant- or animal-derived matter. Inorganic are sometimes called synthetic fertilizers since various chemical treatments are required for their manufacture.
Single nutrient ("straight") fertilizersEdit
The main nitrogen-based straight fertilizer is ammonia or its solutions. Ammonium nitrate (NH4NO3) is also widely used. Urea is another popular source of nitrogen, having the advantage that it is solid and non-explosive, unlike ammonia and ammonium nitrate, respectively. A few percent of the nitrogen fertilizer market (4% in 2007) has been met by calcium ammonium nitrate (Ca(NO3)2 • NH4 • 10H2O).
The main straight phosphate fertilizers are the superphosphates. "Single superphosphate" (SSP) consists of 14–18% P2O5, again in the form of Ca(H2PO4)2, but also phosphogypsum (CaSO4 • 2H2O). Triple superphosphate (TSP) typically consists of 44-48% of P2O5 and no gypsum. A mixture of single superphosphate and triple superphosphate is called double superphosphate. More than 90% of a typical superphosphate fertilizer is water-soluble.
The main potassium-based straight fertilizer is Muriate of Potash (MOP). Muriate of Potash consists of 95-99% KCl, and is typically available as 0-0-60 or 0-0-62 fertilizer.
Multinutrient fertilizers
These fertilizers are common. They consist of two or more nutrient components.
Binary (NP, NK, PK) fertilizers
Major two-component fertilizers provide both nitrogen and phosphorus to the plants. These are called NP fertilizers. The main NP fertilizers are monoammonium phosphate (MAP) and diammonium phosphate (DAP). The active ingredient in MAP is NH4H2PO4. The active ingredient in DAP is (NH4)2HPO4. About 85% of MAP and DAP fertilizers are soluble in water.
NPK fertilizers
NPK fertilizers are three-component fertilizers providing nitrogen, phosphorus, and potassium.
NPK rating is a rating system describing the amount of nitrogen, phosphorus, and potassium in a fertilizer. NPK ratings consist of three numbers separated by dashes (e.g., 10-10-10 or 16-4-8) describing the chemical content of fertilizers.The first number represents the percentage of nitrogen in the product; the second number, P2O5; the third, K2O. Fertilizers do not actually contain P2O5 or K2O, but the system is a conventional shorthand for the amount of the phosphorus (P) or potassium (K) in a fertilizer. A 50-pound (23 kg) bag of fertilizer labeled 16-4-8 contains 8 lb (3.6 kg) of nitrogen (16% of the 50 pounds), an amount of phosphorus equivalent to that in 2 pounds of P2O5 (4% of 50 pounds), and 4 pounds of K2O (8% of 50 pounds). Most fertilizers are labeled according to this N-P-K convention, although Australian convention, following an N-P-K-S system, adds a fourth number for sulfur, and uses elemental values for all values including P and K.
Micronutrients
The main micronutrients are molybdenum, zinc, boron, and copper. These elements are provided as water-soluble salts. Iron presents special problems because it converts to insoluble (bio-unavailable) compounds at moderate soil pH and phosphate concentrations. For this reason, iron is often administered as a chelate complex, e.g., the EDTA derivative. The micronutrient needs depend on the plant and the environment. For example, sugar beets appear to require boron, and legumes require cobalt, while environmental conditions such as heat or drought make boron less available for plants.


Very clear and descriptive article indeed, despite I’m an orthodox supporter/practitioner of Bio-organic farming