There are mainly two types of chemical compounds.
Saturated Compounds
Unsaturated Compounds
Saturated Compounds
First one are Saturated compounds which has signle bond and their bond are completed and their bond capacity is full.
Example: Alkanes are Saturated molecules as they have sigle bond in their structure e.g Methane, Ethane and so on
Formula of Methane CH4. As Carbon has tetra valency thus in methane Carbon has four bonds with hydrogen atom. They give mostly Substitution Reactions.
Unsaturated Compounds
In this type of compounds double are triple bonds are presents.
Example alkenes and alkynes.
Ethene
These compounds mostly prefer Additional Reactions.
Test for Saturted and unsaturated Compounds.
By bromine water test we can tell that the compound is saturated or unsaturated.
If the Compound is saturated then the bromine color remain same i.e Brown color not change
CH4+Br2-> no color changes(no reaction)
If the compound is unsaturated then brom8 color will change. Bromine become color less
C2H4 + Br2- -> Color changes
Benzene
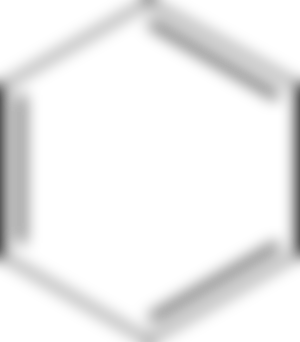
Benzene Structure shown above tell us that Benzene is unsaturated Compounds as it has double bond in its structure. But it behave as saturated compound as its double bonds are not true bond and behave as a single bond. Thus benzen give substitution reaction easily.
Substitution reaction
Those reaction in which one atom is replaced by the other in the compounds.
There are two types of substitution reaction
Electrophilic Substitution reaction
Nucleophile substitution reaction
Benzene mostly give electrophilic Substitution reaction easily.
In electrophilic Substitution reaction of benzene an electrophile is generated and replaced by the one of the hydrogen (proton) of benzene.
What is an electrophile.
Electrophile is a species carrying a positive charge. H+, CH3+ etc
Reaction of Benzene.
Nitration
In nitration a nitro group place the hydrogen in the presence of HNo3 and H2SO4 which creates a NO2+ for replacement.
Too be continued.
This is my first article


You're article is good ,the explanation is good, your good writer