I will try to get one of these posts up every week eventually but every two weeks for now is probably more realistic so we will move on to more interesting stuff eventually!
In the previous post we mentioned atoms but focused on the molecule mainly, which is a collection of atoms arranged in a certain way.
We also learned about how small differences in the number of and ratio those atoms can change a molecule significantly.
Today will focus more closely on the atom itself to see what is going on in there (as much as we think we know).
As we learned an atoms is the smallest unit which one can find an element in and as such are the pieces that can be arranged in various ways to make the Universe as we know it and everything within.
An atom consists of protons, neutrons and electrons. These are known as subatomic particles.
Of these, protons have a positive charge, neutrons have no charge and electrons have a negative charge.
If we look at the periodic table we can discover how many of these each atom has. This is defined by the atom’s atomic number which you will see above the element which you look at in the periodic table.
For example the first element on the periodic table is Hydrogen (H) and the number above it is 1. This tells us that Hydrogen has 1 proton and 1 electron because an atom has no overall charge (it is neutral) the electron’s negative and proton’s positive charge cancel one another out. (There are exceptions to this but we will cover those eventually)
Moving down to the third element, Lithium (Li) we see that it has 3 protons and 3 electrons as indicated by it’s atomic number.
The next one down, Sodium (Na) has 11 protons and 11 electrons and so on.
(Note the periodic table is mostly read from left to right just so you know, these were just given in this order as examples).

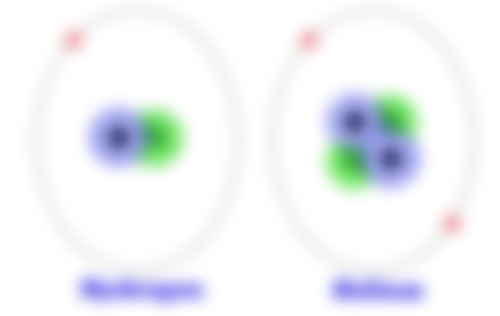
Look at the image above (source) of a hydrogen and a helium atom.
Note the number of subatomic particles.
Hydrogen has 1 proton, 1 neutron and 1 electron
Helium has 2 protons, 2 neutron and 2, electrons
(Helium is number 2 on the periodic table hence the 2 protons and electrons)
The number we find below each element in the periodic table is called it’s Mass Number.
The Mass Number tells us how many total protons and neutrons together there are in the atom.
Using the Mass Number and Atomic number we can easily calculate the number of neutrons in the atom alone. We can simply take away the Atomic Number (the top one) from the Atomic Mass (the bottom one).
We sometimes need to round up the Atomic mass to the nearest whole number for our calculations so for example and atomic mass of 15.999 (Oxygen) becomes 16.
Looking at the periodic table again we can use Bromine (Br) (try to find it by it’s number, hint; its over on the right side of the table). We can see it has an Atomic mass of 80 and an atomic number of 35.
As before Atomic Mass – Atomic number = Number of neutrons so we have 80-35 = 45 neutrons!
In the atom itself, the protons and neutrons are found together in an area called the Nucleus and the electrons orbit the nucleus like satellites around the Earth.
These electrons are involved in interactions between atoms and molecules as their negative charge makes them attracted to positive charges on other molecules or atoms and repelled by the negative charge on others.
If two atoms or molecules share electrons strong bonds can be formed other times it is just simple attractive force between two molecules like if you hold two magnets near one another with the positive and negative ends facing.
We will learn more about chemical bonds soon as there are a few types.
I hope you enjoyed this, any questions or comments leave them below!