
image source : synoxis
Stories that certain foods can cause acidity in the body, and thus cancer, are stories for young children of those who either have nothing to do with physiology, chemistry, pH regulation or those malicious, who want to take advantage of the sale of drugs that claim to raise pH. alkaline.
There are also some really incredibly untrue, not to say stupid, tables in which it is claimed that lemon is of a basic character, and dandelion even has a pH value of 26. Anyone who would take a pH-meter or ordinary litmus paper could determine that lemon juice of acidic character, which is also shown when water from rose petals is added lemon juice - it turns red, due to anticyclonic from rose petals which are an indicator of pH value. Anyone who knows anything about chemistry, and this is really elementary school chemistry, knows that there is no pH 26, but that it is a scale from 0 to 14.
Also, the cause of cancer is not acidity

The body has amazing systems of regulation of a favorable state, balance, which we call homeostasis. We cannot live outside of homeostasis. If our temperature regulation is disturbed - it is bad for us. The same with the acid-base status of the organism, blood sugar and many other parameters. Fortunately, the body has systems that regulate it. One of the systems of regulation of acid-base status is breathing. It also regulates the pH value of blood, which ranges from 7.35 to 7.45. Although this is only slightly higher than the neutral value and on the base side of the pH scale, 7.35 is considered acidosis (although narrowly it is not an acidic pH, but only a “more acidic” value than pH 7.4). 7.45 is the value going towards the alkaline side.
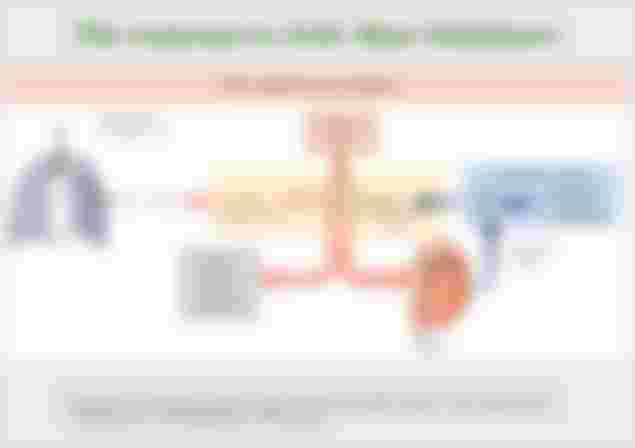
The respiratory system contributes to the balance of acids and bases in the body by regulating the level of Carbonic acid in the blood. CO2 in the blood reacts easily with water because there is an enzyme carbon anhydrous that accelerates and stimulates this reaction in which Carbonic acid is produced and the levels of CO2 and Carbonic acid in the blood are in balance. In other words, they pass into each other, when there is an excess of one:

image source : Human biology online
When the level of CO2 in the blood rises, for example when you hold your breath, excess CO2 reacts with water and produces additional Carbonic acid, lowering the pH in the blood. Increasing the speed, ie. breathing depth (which you can feel the “urge” to hold your breath, try to do it - something will make you hold your breath) allows you to exhale more CO2. The loss of CO2 from the body reduces the level of Carbonic acid in the blood and thus adjusts the pH to normal levels. This system also works in the opposite direction. Excessively deep and rapid breathing (as is the case in hyperventilation) removes CO2 from the blood and lowers Carbonic acid levels, making the blood too alkaline. This short alkalosis can be removed by re-breathing the air exhaled into a paper bag (“hyperventilation bags” in case of panic). Re-inhaling exhaled air will quickly lower your blood pH to normal.
Chemical reactions that regulate CO2 and Carbonic acid levels occur in the lungs when blood passes through the pulmonary capillaries. Smaller breathing adjustments are usually sufficient to adjust blood pH by changing the amount of CO2 exhaled. Doubling the breathing rate in less than a minute, removing “extra” CO2, would increase blood pH by 0.2 parts of the scale. This situation is common if you exercise hard for a while. To maintain the necessary energy production, the body produces excess CO2 and lactic acid if you exercise above your aerobic threshold, which you will later feel as muscle pain, due to the synthesis and accumulation of lactic acid. To balance the increased acid production, the respiration rate is increased which prevents acidosis.
The body regulates the rate of respiration using chemoreceptors, which primarily use CO2 as a signal. Peripheral blood sensors are located in the walls of the aorta and carotid arteries. These sensors signal the brain to immediately adjust its breathing rate if CO2 levels rise or fall. Changes in pH affect the respiratory center in the medulla, which can directly modulate the rate of respiration to bring the pH back to the normal range.
Hypercapnia or abnormally elevated levels of CO2 in the blood occur in any situation that impairs respiratory function, including pneumonia and congestive heart failure. Decreased breathing (hypo-ventilation) due to medications such as morphine, barbiturates, or ethanol, or even just holding your breath, can also result in hypercapnia. Hypocapnia or abnormally low levels of CO2 in the blood occur in the case of hyperventilation when CO2 is rejected, such as when the room temperature is elevated, in fever or in cases of hysteria.
From this we see that the regulation of the pH value of the blood is a very subtle mechanism, and those who say that masks cause acidosis really do not know the physiology and will not know it. The reduction of the partial pressure of oxygen during prolonged wearing of masks is up to about 2%, which is not enough to cause the effects they are talking about, least of all acidosis. Which, I repeat, is not the cause of cancer.

🙂