Inert gas and its stability: -
In the periodic table chapter, they have learned the details of inert gas i.e. group 14These Also gained ignorance about electron configuration. Yet here they show their electron configuration.
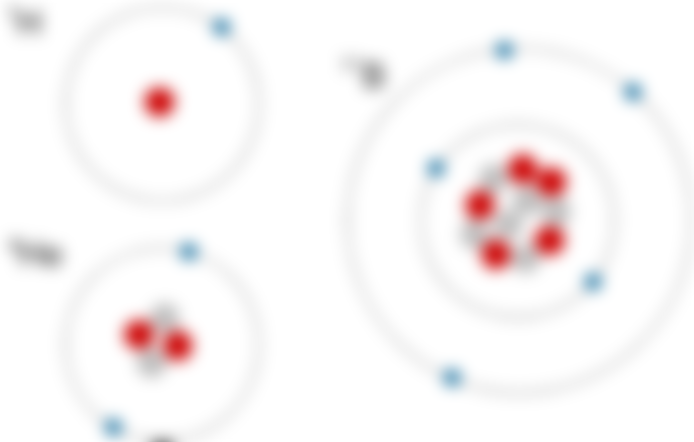
The electron configuration of inert gases shows that the last energy level of helium contains T electrons. There are. Helium needs 5 electrons to make up for its ultimate energy, so this The electron configuration is stable.

He (2) Ne (10) Ar (18) Kr (36) Xe (38 in (86) —Electrons exist. If there are 4 electrons in the last energy level of an element in a corner, they are maximum Achieves stability. If there are 2 in the last energy level, it is called double and if there are 4 electrons Call him octave. The last energy level is higher than the inert gases due to double and octal fullness Is stable. Inert gases electron at other angles in inert gases due to greater stability Does not provide.

Tries to achieve electron configuration. When two atoms of the same element or different elements are close When positioned they receive, reject or share electrons at their latest energy level Achieves electron configuration of inert gas. This creates a kind of attraction between them Yes, that attraction is what we call chemical bonding. So it can be said that chemical bonds are formed.
It does not even receive electrons from other elementsectrons from the external energy of a neutral atom.

We know that, under normal conditions, the nucleus of an atom has as many positive charges or positive keys. Has protons and has exactly the same negative charge or negative at different energy levels outside the nucleus Contains charged electrons. This results in the atom being neutral or charge neutral. Like this A charge is the removal of one or more electrons from the external energy of a neutral atom

Now the question is why the atoms did not exist separately? Why are they molecules attached to each other Made? In the meantime, they have learned that every element is stable at the latest energy level.......



camistiy is the most subject of science. My good subject chemist, i will the were of information technology minister and follow me written and follow this better. Most content witter in cemesty subjects. We have good earning site of its.