Assalamualaikum everyone. I am the writer will give Explanation of H-spectrum from Bohr's Atom Model today.
First of all again saying that as i am a chemist so you will get lot more articles on chemistry in my profile..so get ready to know about chemistry. I am just writing article on some basic knowledge. As i have already written an article " Before you enter the world of chemistry. This article is also an part of that. Let's start,
Explanation of H spectrum
The creation of the atomic spectrum of H can be explained from the Bohr model. Even though the H atom has only one electron, many lines are formed in its atomic spectrum. This is because the hydrogen molecule (H) first turns into an atom (H) under the influence of high electrical energy. The electrons in the numerous atoms of hydrogen then absorb different amounts of energy from the source and rise to different high energy levels. These more powerful layers at infinite distances from the nucleus are called excited states.At high energy levels the electrons are not stable. When the energy source is removed, those electrons can jump back to the same low energy level by radiating light energy from different high energy levels. Low energy level is low energy and permanent, it is called minimum energy state (ground state).
The resulting lines are of different lengths along the spectrum. Various scientists have discovered the image of the line spectrum of H. They have different names for the line spectrum of H, such as (1) Stimulated electrons at different high energy levels. The lines found in the H-spectrum when the energy radiates back to the 1st energy level are called the Lyman series. Says. Similarly, the return of electrons from high energy levels to 2nd, 3rd, 4th and 5th energy levels results in a spectrum. Respectively,
Lymen series
Bamar series
Paschen series
Bracket series
Pfund series.

Image source- https://socratic.org/questions/how-does-bohr-s-model-of-the-atom-explain-the-line-spectrum-of-hydrogen
Rydberg equation
The number of waves of different lines in the hydrogen spectrum is found to be from the following Reidberg equation👇
1/lambda= RH( 1/nf^2 - 1/ni^2)

where RH is 1.097 x 10^7 m^-1 and the principle quantum numbers of n2 is greater than n1.
The line spectra resulting from the transfer of electrons from different layers are shown
The electrons of the hydrogen atom return from different high energy levels 2nd, 3rd, 4th, 5th, 6th, 7th level i.e. n = 2, 3, 4, 5, 6, 7 to low energy level n = 1. The transfer of electrons from the 5th, 6th, 7th energy level i.e. n = 3, 4, 5, 6, 7 etc. to the 2nd energy level n = 2 produces a Balmer series in the visible region. Similarly, the transition from 5th, 6th, 7th power level, 112 = 5, 6, 7, 6 ...... to 3rd power level ii) = 3 in the infrared region Paschen Series, 6th, 7th, 8th energy level, n = 6, 7, 8.... to 4th energy level n=4 and the electron returns from the 7th, 8th ..... energy level n2 = 7, 8 .... to the 5th energy level n = 5. Is observed. The values of nj and n in different series are as follows
In the Lymen series-n1 = 1; n2 = 2, 3, 4, 5. 5....alpha (Ultraviolet region)
In the Balmer series- n2 = 2: n2 = 3, 4, 5, 6.....alpha (Visible area)
In the Paschen series - n3 = 3; n = 4, 5, 6, 7... alpha( Infrared areas)
In the bracket series n4 = 4; n = 5, 6, 7, 8....alpha [infrared region]
In the pfund series- n5 =5; n = 6, 7, 8, 9....alpha [ Infrared area]

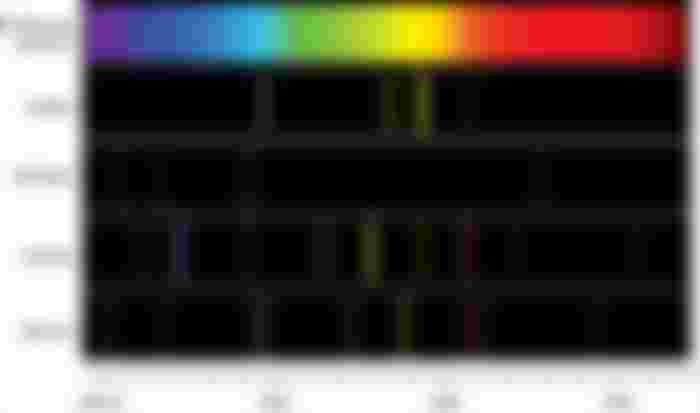
Element detection from Line Spectrum: Students' Activity
Like the 'fingerprint' of every human being, the line spectrum of atoms is different. (1) So every element has a characteristic spectrum. (ii) Each line of the spectrum has a specific wavelength. So the element can be identified by looking at the color and wavelength created in the line spectrum.

For example,
The spectrum of sodium atoms consists of two fine yellow lines separated by black space or individual. The wavelength of the first line (D) between the two lines = 589.6mm and the wavelength of the second line (D), ^ = 590.nm
The 4-line spectrum of the hydrogen atom is visible in the range of visible light; (1) Blue forest = 4.10.1nm. 434.1 nm 486.1 nm (blue) and red = 656.3mm
Similarly, among the many line spectra of Mercury, green = 540nm, orange = 630 nm And the lines of red = 680 nm in length are clear
Among the many Betha spectra of strontium (Sr) the sky color = 468nm 465nm 476am, four lines of 490 nm and red = 669nm, 678nm. Three lines of 715mm can be seen.
Finishing
So, this is not so hard to study. This is such simple things we aften get tensed that how can we memorize them. It will be much easy only if you Understand the topic. I tried to explain easily but if you have any question to ask then feel free to ask me. I will try to answer your question.
Image sources
Lead image - https://courses.lumenlearning.com/physics/chapter/30-3-bohrs-theory-of-the-hydrogen-atom/
Till then, stay blessed, eat healthy be strong and keep smiling always❤️

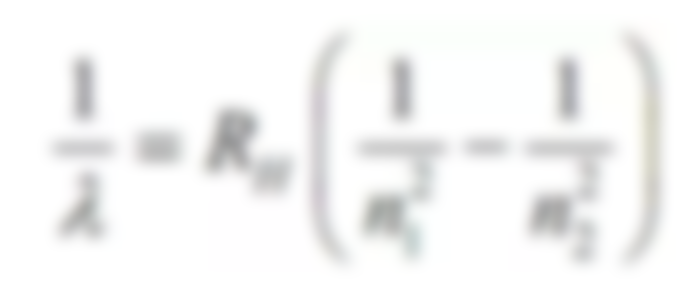

I am so dull in chemistry... But I have a high interest in this subject... H-spectrum is a very difficult concept... But you write very well... Now the concept is more clear to me...