Sulfur is everywhere
Inktober is done and chemist Hanzell is making a comeback~~!!! I was supposed to do my comeback on the first but my sleepiness had other plans. Plus, I've been busy making plans with someone on hive UwU
Alright, so since I already wrote about ore types, processing and a few other terms before, I thought now would be a good time to talk about some common metals/elements that usually come through the lab doors.
So I talked about sulphides and sulphates before, but what about thiosulfates and the native sulphur ore itself?
Yes! I’ll be talking about sulphur/sulfur because it is one of the most common ore types we get in our lab. It’s also one of the most used ions in the lab too.
So the element sulfur
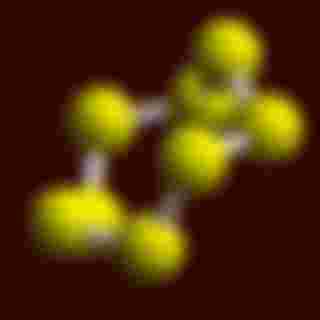
It has an atomic number of 16 with an atomic weight of 32.01 g/mol. It has the most number of allotropes (element with different forms/phases. Think carbon powder, graphite, diamond. All carbon, just different atomic arrangements. Same logic with sulfur) yet its most stable allotrope is the orthorhombic sulphur (S8).

here's the crystal structure of the orthorhombic sulfur. This is just a 3D model I got from here: https://www.webelements.com/sulfur/crystal_structure.html
It’s a poor conductor of anything, but it’s one of the most reactive ions out there. It can combine with almost any element on the periodic table except for gold, platinum, and inert gases since these aren’t reactive to begin with.
For occurance. It is the 10th most abundant element in the universe because it’s not only found on land/earth, but in the oceans, in the lithosphere and meteorites too. But on a matter of where you’re most likely to find sulfur, I suggest near volcanoes. It can occur in its native form (this you can find in sedimentary rocks), which is a pale yellow, soft solid/crystal

another name for native sulfur is also brimstone and I think it's really pretty if it weren't so hazardous XD
This is what they use to make sulfa-drugs and those sulfur beauty products like sulfur soap
source: https://www.geologysuperstore.com/product/brimstone-elemental-sulphur/
Or it can occur mixed in with other metals, and that’s what I’ve been talking about for a while now.
I think, ever since I’ve started working in labs, sulfur has always been an analyte we needed to quantify. Like when I tested coal, we had a sulfur analyzer because combusted coal produces sulfur gasses that in turn, contribute to acid rain and eutrophication, so we had to see if the coal sample we received would be good for energy production.
Meanwhile, when you look at food, it’s seen as a preservative, namely as a sulphite (SO2) which is a gas blown into dried fruit packages like prunes, raisins, etc.. It’s to ensure that moulds won’t grow on fruits since sulphites do act as an antimicrobial agent here.
As for when I handled fertilizers, sulfur is seen as a nutrient in this case. Going back to the eutrophication effect it has, sulfur can promote algal growth as well as plant growth. That’s why back when I handled fertilizers, we did gravimetric sulfur analysis because of the sulfur percentage in just 1 gram sample most of the time.

this is probably the last batch of sulfur I digested in my previous company and it was also the dirtiest because I think it was too high in phosphorus so it came out colored like that.
Now that I’m back in the minerals industry, sulfur is back to being a contaminant XD but now, checking for sulfur is kind of like a preliminary check we do to the oores, kind of like when we run all the samples through a four acid digestion as a preliminary check. In the case of the sulfur check though, it’s sort of the same thing with coal. Sulfur gas does form when you process ores through roasting or calcination.

roasting and calcination from the geochemsitry pdf my supervisor gave me. Sorry, I'm so lazy to look for the actual source ;;w;;
Then here is the occurance of sulfur in ores:




it's quite a long list, and that's not even all of it. Although we've sampled and tested quite a number on that list, but there's still quite a bit of ores left for me to explore.
There’s also the fact that if the sulfur content is high, and if it happens to be a sulfur ore of high purity then it can be used for plenty of other things, specifically in the prooduction of sulfuric acid and in the pharmaceutical side of things since native sulfur is considered one of the oldest fungicides, pesticides, and antimicrobial agent.
Sources:
https://doi.org/10.1016/B0-12-369397-7/00583-5
https://geology.com/minerals/sulfur.shtml
https://www.mindat.org/min-3826.html
I wanted to dive into how sulfur ores are processed and the analyzer we have for it eventually. When I can get an idea of how I'll be explaining combustion chemistry properly.